ISO Registration
ISO registration is ideal for the purpose of enhancing the standard and quality of the organization’s services or products.
ISO refers to the International Standard Organization. ISO certification provides credibility to the organization. In the global marketplace, to maintain consistency and quality across industries and nations, checks and balances need to be in one place. Certification ensures that the management system, the manufacturing process has all the requirements for standardization and quality assurance. ISO certification exists in many areas of industry like medical devices, energy management, and social responsibility. It is an independent, non –governmental, international organization with a membership of 164 national standard bodies.
The purpose of ISO is to form certain standards that will guarantee the quality, safety, and efficiency of the products, services, and systems. ISO registration also acts as a proof that the manufacturing process, service or the documentation process, management system are pertaining to all the requirements related to standardization and quality assurance.
ISO certificate is issued to many industries, that is, from energy management and social responsibility to medical devices and risk management.
Every ISO certification has a separate set of standard and criteria, and even it is classified numerically. For example, ISO 9001:2015.
ISO 9001 Certification Meaning
A company that fulfils all the requirements set forth in the ISO 9001 Standard is eligible to receive ISO 9001 certification. ISO 9001 certification is used for a quality management system. Organizations follow the standards to maintain the consistency in their products and services so that their customers’ and regulatory requirements are met on time and keeps on improving.
ISO registration defines the essentials that are needed to be adopted for a quality management system. It covers information like planning, documented data, and process interaction. It also includes any important notification related to resource management like human resources. ISO involves in various activities like internal audits and corrective & prevention plan. It measures, improvise, and analyze the QMS on the basis of activities it conducts.
Benefits of ISO Certification
Obtaining ISO 9001 is important for food manufacturing units. ISO 9001 certificate assures the quality in products is maintained. Small to medium-sized manufacturer (SMM) need ISO 9001 certification to measure their QMS. Having a QMS allows you to keep a check on the irregularities for food safety standards and also helps in delivering high quality products.
- Helps in improving customer satisfaction, because ISO standards intend to make organizations deliver best quality services to their customers.
- If the company is looking forward to expand their business internationally, certification plays a very important role to build credibility in overseas business.
- Even for the purpose of govt. Tenders ISO certification plays an essential role to bid in tenders.
- After obtaining the certification, the product should match the quality of international standard; else the product will face the rejection on the grounds of quality issues.
- Maintaining standards in food products yield growth, profitability, and cost savings for SMMs.
Documents Required for Online ISO Registration
- Copy of PAN Card
- Passport size photograph
- Copy of Adhaar card/Voter ID
- Two copies of Sale Purchase Invoice
Types of ISO Certificates
- Copy of PAN Card
- Passport size photograph
- Copy of Adhaar card/Voter ID
- Two copies of Sale Purchase Invoice
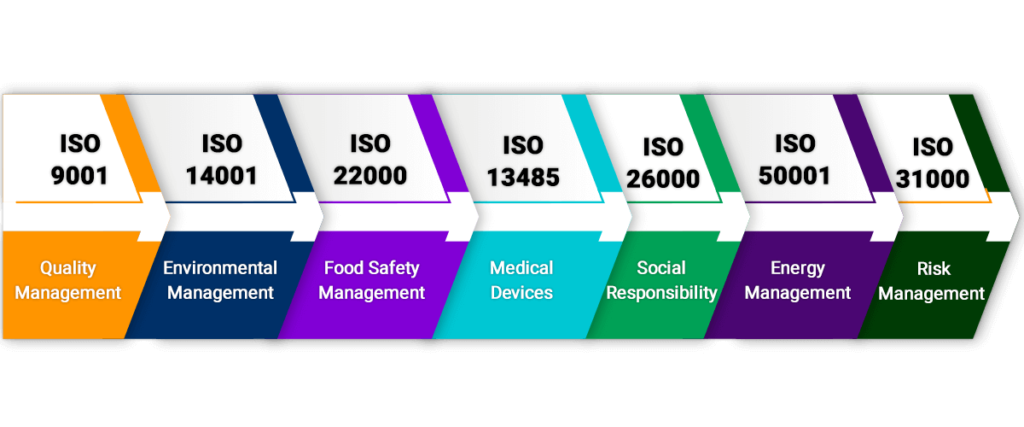
ISO 9001-Quality Management
This certificate sets particular criteria for a quality management. Although it is not necessary to set any criteria but many organizations choose to use it because it helps in creating customer focus thus creating a market place for the brand.
ISO 14001- Environmental Management
This one is created keeping the environment in mind it sets out a criteria that every organization must follow to check the environmental management system.
ISO 22000- Food Safety Management
This certificate helps in safeguarding and identifying the food safety Standard hazards. Number of organization use this and sets out a particular criteria for their organizations to comply with.
ISO 13485 Medical Devices
Organizations that are involved in designing, production, installation, providing services related to medical devices use this certificate. The purpose of this organization is to make sure that organizations are adhering to the best quality management system and best practices.
ISO 26000 – Social Responsibility
Any organization in the society has a huge responsibility to protect and prevent the future of people like us. This certificate sets a particular guidance for such organizations and also tells them how to perform in an ethical and transparent way that will contribute to the better health and society.
ISO 50001 – Energy Management
Organization willingly prefers to go with this standard because it is crucial to harmonize crucial management with their efforts to improvise environment management and quality.
ISO 31000 –Risk Management
To work securely and with the expert reputation, it is important to deal with the hazard adequately which encourages the associations to perform well and with full sureness. This Certification gives rules, standards, system and a procedure to manage the hazard. It very well may be utilized by any association paying little heed to their business size or activity.
Importance of ISO Certification
- Boost Consumer Confidence
It increases the trust and confidence level of the users, when consumers see an international logo they tend to trust the product or service more.
- Involvement Of Consumers
Consumers are involved for the way toward making and composing International Standards that are as wide as could reasonably be expected.
- Broaden The Market
It helps in growth and expansion of business in international market.
Registration Procedure for ISO
Application To Certification Body
Business man needs to make an application as prescribed by ISO Certification body. The application must contain the rights and obligation of the businessmen.
Document Evaluation By Registrar
Now, registrar of certification body will evaluate the documents of quality manuals and other documents like policies and procedure which company is following.
Inspection Or Audit In Company
Registrar will now conduct an onsite inspection, to check what all necessary changes are required in the company. If registrar observes anything not meeting with the requirement of ISO standards, it is the duty of businessman to come out with an action plan to eliminate all the flaws of the company. The action plan needs to contain the list of all the tasks which are required to be performed.
Final Audit
The final audit is re-audit of the affected area because registrar cannot proceed until all the significant non-conformities gets verified.
Get ISO Certificate
When all the conformities are addressed, findings will be updated in the ISO reports, and certificate will be granted.
Surveillance Audits
Such audits will be performed from time to time with the aim to check whether a company is maintaining the ISO standards properly.

Improves Staff Performance And Overall Productivity
Employees are motivated to follow the process that will help in maintaining quality and also proves helpful in quickly identifying and resolving the problem.
QMS is a proper system that contains procedures, documents and also defines responsibilities, processes and objectives. It is a blueprint of all the requirements that SMM need to succeed.
Flesh Out Your Company’s Processes
ISO 9001 is fragmented without having a complete and defined business process. It should also define measures to be taken for quality control and responsibilities of all the employees.
Make sure that your QMS also guarantees on-time delivery and accurately reflect your system’s performance.
Reduce Waste And Improve Efficiency
The purpose of drafting an effective QMS is to keep an eye on preventive measures, so that wasteful problem can be avoided completely for the future. Certification helps in the process of continuous improvement so that you can always seek to reduce waste and improve efficiency.
BIS Registration
BIS is a national body which ensures the quality, safety and reliability of products in India. The purpose of BIS is to examine the samples of products when they are preliminary and surveillance stage. India has a total of eight central, four regional and three branch laboratories of BIS.
Government Of India Encourages BIS Registration Because Of The Following Reasons:

BIS Registration Product Scheme
BIS Registration product Scheme is one of the largest schemes all over the world with over 26500 licenses covering more than 900 products. It allows the manufacturer to use the popular ISI Mark on their products which says that the product is pure and healthy. BIS Registration product scheme also operates Foreign Manufacturers Certification Scheme under which overseas manufacturer can be granted a license to use the BIS standard mark.
Benefits of BIS Registration for manufacturing units
It is crucial for the manufacturer to obtain BIS Registration as it involves the following factors-
- It provides the safeguard to the public health
- It provides the quality assurance of the product
- It protects the consumer from dangerous products
- It also increases the confidence among the customers.
Till now, over 350 BIS Registration have been granted for over 50 Indian standards in 40 different countries.
Documents Required for BIS Registration
Documents that you need to provide for BIS registration process is divided into two parts:
First part talks about the technical information of the products available for lab test. The information manufacturer has to submit is the construction details of the product like:
- PCB Layout
- Schematic Diagram
- User Manual
- Critical Components List
In the second part you have to submit the Factory Documents & Information to complete the BIS Application Form and Process, that is, all basic information related to the manufacturing unit:
- Trademark Registration Copy
- Organizational chart of the factory
- Legal address proof of factory
- List of machinery
- List of equipments
- Documents of Authorized Indian Representative (AIR) when the manufacturer is from outside India
The BIS Registration procedures will take approximately 30-35 days to complete including testing time of 15-20 days and the BIS Application processing time which is 10-15 days.
Types of BIS Registration Schemes
The BIS Registration for the product is categorized under different types of schemes which is as follows-
Normal Procedure For Domestic Manufacturer
The applicant needs to submit the BIS Registration or BIS Registration application along with certain documents and the requisite fees. After submitting the application an evaluation is carried out by a BIS officer where the samples are tested in the factory and drawn an independent testing report. And, if all things go well BIS Registration is provided where the sampling procedure is acceptable. BIS Registration is granted within 4 months after the submission of the application.
Simplified Procedure for Domestic Manufacturers
In the simplified procedure, the applicant along with the application for BIS Registration submits a test report of the sample from the approved lab to the BIS officer. If the test report is satisfactory then the BIS officer will verify the factory and grant certificate of registration within 30 days of submission of BIS Registration application with a required test report.
The simplified procedure is applicable for grant of license of All India First license cases and for all the products except for the following:
- Products like cylinders, valves, cement, etc. , where it is necessary to get the approval from another statutory authority;
- All those products for which BIS license is issued on the basis of factory testing;
- Packaged Drinking Water (PDW) and Packaged Natural Mineral Water (PNMW).
Eco Mark Scheme
BIS also provides License to environmentally friendly products under a special scheme- ECO Mark Scheme where all environmental products are categorized conforming to the basic requirements under Indian Standards.
Foreign Manufacturers Certification Scheme
The ISI mark for overseas applicants or manufacturers is specially designed and granted under a separate scheme within 6 months period. In the year 2000 the concept of Foreign Manufacturers Certification Scheme (FMCS) was introduced by Bureau of Indian Standards (BIS) with the aim to enable the overseas applicants or foreign manufacturers so that they too can use Standard Mark on their products. A BIS license that falls under this procedure can be obtained in 6 months.
Registration Procedure for BIS Registration
Licensing Process
In the licensing process, the following steps need to take-
- There will be an application submission
- Then the BIS Officer will go for a preliminary Inspection
- After the Inspection, the samples will be tested
- After completing all the evaluation, the final result will be derived
Surveillance Process
After the Inspection Process, the next process is called the surveillance process in which the factory’s complete survey is conducted. The following are the steps taken for the surveillance process-
- The Inspecting authority will visit the factory and testify it
- After the testing, the samples were taken
- The samples will be sent to the Independent labs
- The test report will brief the complaint or give feedback on the investigation
- The performance review report will be ready
- Once the report is reviewed license is granted
BIS Registration Mandatory Products
Not all electronic products or other products need BIS Registration but there are certain products that falls under the mandatory requirement. The list of products is mentioned below-
- Cement and Concrete
- Diesel Engines
- Household electronics
- Food and related products
- Oil Pressure Stoves
- Automobile Accessories
- Steel products
- Wood Products
- Leather Product
- Cylinders, valves, and Regulators
- Medical Equipment
- Electrical Transformers
- Testing instruments
- Rubber and plastic
- Machinery and equipment
Validity for BIS Registration
The BIS Registration will be valid for two years and can be renewed if the product and the standard are not changed. License renewal is done for the minimum period of one year and maximum up to five years. Though, the renewal depends on the annual license fee and advance minimum marking fee. In case the application for renewal is made after the validity of the license, the applicant has to pay late fee of rupees five thousand.
What is Compulsory Registration Scheme (CRS)?
On October 2012, the Ministry of Electronics & Information Technology came with an order that says no one is allowed to manufacture or store certain goods. It issued “Electronics and Information Technology Goods (requirement for Company Registration) Order, 2012 and has given 15 categories of electronic products that are exempted from sale, import or distribution. Notify goods do not comply with the Indian Standards. Thus, it is essential for the manufacturer of the listed electronic products to apply for BIS registration by following the prescribed procedure:
- Bureau of Indian Standards (BIS) at that point enrols the manufacturers under its registration scheme.
- The enlisted makers are permitted to declare that their products agree to the prescribed Indian Standards.
- The registered manufacturers then have the authority to mention that their products use the Standard Mark notified by the Bureau.
- Provisions of Chapter IVA of BIS Rules 1987, regulates Compulsory Registration Scheme.
Ministry of Electronics & Information Technology (MeitY) has expanded its list by adding 50 more products, all these products fall under the category of products that need Compulsory Registration.
Who can apply?
- Manufacturers of the products notified in the scheme which is located in India or outside India.
- The separate Registration number for products being manufactured at different locations.
- The separate Registration number for each brand being manufactured at the same location.
National Accreditation Board for Testing & Calibration Laboratories (NABL)
National Accreditation Board for Testing & Calibration Laboratories (NABL) is a society providing Recognition (Accreditation) of Technical competency of a testing, calibration, medical laboratory & Proficiency testing provider (PTP) & Reference Material Producer (RMP) for a selected scope of standards.
It is an autonomous body under the Department of Science and Technology having Mutual Recognition Arrangements with APLAC (Asia Pacific Laboratory Accreditation Cooperation) , MRA (Mutual Recognition Arrangement) , ILAC (International Laboratory Accreditation Cooperation) and is registered under the Societies Act., with the objective to provide Government, Industry and Industry Association in general for third-party assessment of the quality and technical competence of testing and calibration laboratories.
NABL Accreditation
Accreditation is the attestation by third party with the conformity assessment body convincing the formal demonstration of its ability to hold out specific conformity assessment task. Conformity Assessment Body (CAB) does the testing which includes medical/standardisation Laboratory, Proficiency Testing supplier, Certified Reference Material Producer.
The liberalization of trade and industry policies of Government of India has created consciousness among domestic trade and providing greater thrust for export. As a consequence testing centres and laboratories have to show the capabilities acceptable at an international level of competence.
It is a method by which the body gives recognition, based on third party assessment and as per international standards of a technical competence for specific tests/ measurements.
In the same manner Proficiency testing Provider accreditation gives formal recognition of competence for organizations that provide proficiency testing. Based on the third party assessment Reference Material Producers Accreditation gives formal recognition of competence following international standards to carry out the production of reference materials.
Scope of NABL Accreditation
NABL Accreditation is currently being given in the following fields and disciplines. Depending upon the scope discipline belongs to the multi-disciplinary. CABs shall have to apply in relevant discipline separately. On the scope of accreditation, refer to the relevant specific criteria.
- Testing
- Calibration
- Medical
- Biological
- Photometry
- Chemical
- Radiological
- Electrical
- Electronics
- Fluid-Flow
- Mechanical
- Forensic
- Electro-Technical
- Medical Devices
- Thermal
- Optical
- Clinical Pathology
- Haematology & Immunohematology
- Microbiology & Serology
- Histopathology
- Cytopathology
- Genetics
- Nuclear Medicine (in-vitro tests only
Benefits of NABL Accreditation
Formal recognition of ability of a laboratory by an Associate in accordance with international criteria has many advantages:
- Increased confidence in Testing/ activity Reports issued by the laboratory
- Better management of laboratory operations and feedback as to whether or not they have technically competent and sound Quality Assurance System.
- Potential increase in business because of enhanced customer satisfaction and confidence.
- Customers will search and identify the laboratories accredited by NABL for their needs from the NABL Web-site or Directory of Authorised Laboratories
- Users of authorised laboratories enjoy more access for their products, in both domestic and international markets.
- Savings in terms of time and cash thanks for the reduction or elimination for re-testing of product.
NABL SERVICES AND CONSULTING ARE PROVIDED WHICH INCLUDES TRAINING, DOCUMENTATION, IMPLEMENTATION ASSISTANCE, INTERNAL AUDIT AND ACCREDITATION PROCESS ASSISTANCE.
Given below is the methodology to implement ISO 17025 in your organization.
CreatingAwareness :- Creating awareness about standard and quality management in the entire organization at all Levels.
Policy & Objective :-
- Finalizing policy for quality and objectives as per requirements.
- Quality policy keeping the commitment towards customers and employees in mind.
- On the basis of SMART technique. (Specific, Measurable, Achievable, Relevant and Time bound) Quality objectives are developed.
Gap Analysis:-
- Mapping of your existing operations with that of the requirements as per the standard asked for.
- Preparation of detailed gap analysis report which shows degree of compliance at present level with various clauses and requirements.
Documentation:-
As per QMS assistance in following documents preparation.
- Quality Manual
- Procedures and Functional Formats/ test/system
- Functional instructions (work/ test)
Assistance In UOM And Method Validation
Internal Audit
- Conduct of internal auditor training
- Internal audit Master Plan/schedule/report
- Internal audit result action plan
QMS meeting
- Management Review Meeting its agenda, minutes and action plan
Shadow Audit
- Pre-certification audit to review in line with accreditation body to reflect the changes required as per the document.
- Before the final Certification audit determining and addressing Non-Conformities if any in the system.
Final accreditation Audit
Final accreditation audit will be done by accreditation body. We will assist your organization in closing non-conformities (if any) and after the audit your organization will be awarded ISO 17025 accreditation.
ISI Mark Registration / Certification in India
In India, there are certain products that contain the ISI mark on its packaging, and we use these products in our daily life. But many of us don’t know what the mark truly means.
ISI stands for Indian Standard Institute. ISI mark refers to a certified mark which is used for industrial items sold in India. Industrial items are the items used by a company for the business purpose. It assures that the product which you are buying is safe to use and is of good quality.
What is ISI Mark Registration?
ISI mark registration is mandatory for all the certifying products sold in India. The mark is meant for better quality and safety of the products. The Products having ISI indicate that products are in compliance with the Indian standard quality. Through getting ISI certification on your products, you are eligible to expand your business. If products sold in India don’t have ISI, then those products cannot be sold within the territory of India.
Purpose of ISI Registration
The purpose of ISI registration is to
- Provide quality assurance of any product or good
- Increase the confidence of the customer while buying the good
- Provide a safeguard to the health of the customer
Is it mandatory to obtain ISI Mark?
The government of India, as well as Bureau of Indian Standard, makes it mandatory to obtain ISI on certain products sold in India, product details give below. Those products include electrical appliances as well as industrial items. However, from September 2012 ISI is compulsory for certified steel products and steel items.
Authorized Body to Issue ISI Mark
ISI certifies that a product is made as per the Indian Standard. Bureau of Indian Standards (BIS) is an authorized body for the issuance of the mark.
List of the Products that require ISI Registration
For the following products, ISI registration is mandatory:
- Cement
- Steel products
- Electrical Transformers
- Food Products
- Cylinders, valves, and regulators
- Batteries
- Capacitors
- Electrical motors
- Stainless Steel plate
- Clinical thermometer
- Packaged Drinking water
- Stoves
- Steel wire and steel sheets
- Kitchen Appliances
Benefits of ISI Mark
There are numerous benefits of ISI mark. The list of the benefits of ISI is described below:
- It helps to increase the satisfaction of the customer.
- Where the customer is unsatisfied with the product’s quality, then the company selling the product will exchange the product with a new one.
- For every customer, the mark makes it possible to get the best quality of the product.
- If any customer finds that the product having ISI is of bad quality, then the customer can take action against the manufacturer of the product.
- It helps the manufacturers and owners of the product to increase their business.
Levy of Penalty for Falsification of ISI mark
Where it finds that manufacture of any goods or products counterfeits the registered mark, then a penalty of Rs. 50,000 along with imprisonment up to one year will levy on that manufacturer.
ISI Mark Registration Process
To obtain ISI registration, we have to follow the below steps:
- Choose the product code
- Firstly, you are required to select the product quality as prescribed in the Indian Standard Institute.Identify an ISI standard code for your product
- Filing of Application Form
- After choosing the product code, in the next step, you have to file the registration application form. (Form-V).
- Affix all the necessary documents along with the application form.
- In this step, you need to pay fees as required for the certification and inspection of your factory premise.
- Inspection of the Factory premise
- Upon successful submission of the application, Inspection team and the persons authorized by the Government will visit the factory premise.
- The inspection team and authorized person will inspect the factory premise and quality control process.
- The inspection team will take some samples of your product and good for testing in BIS approved lab.
- Collect the Test report
- Get lab test report of the sample done by the inspection
- Submit the lap report to BIS (Bureau of Indian Standards)
- Issuance of ISI registration certificate
- BIS, after proper verification of the testing report and application form, will issue a registration certificate.
Time Required to get ISI registration certificate
Generally, it takes 30-90 days for domestic manufacturers from the date of submission of an application to get ISI registration certificate, and 90-120 days for foreign manufacturers from the date of submission of an application to get ISI registration certificate
Documents Required To Apply For ISI Registration
If you are willing to take ISI registration, then you have to submit certain documents as described below:
- Registration certificate of the company
- Receipt of Property tax
- Insurance policy
- If the property is rented, then submit the rent agreement
- Telephone bill
- Electricity bill
- Bank statement
- Aadhar card of the directors of the company
- Voter ID card
- Driving License
- Copy of test reports
- List of Manufacturing machinery
- Copies of calibration certificates of testing equipment
